HCRU Study
Study 1: HCRU Study Design1
Methodology
- MBV* was evaluated in the SOLSTICE trial, a multicenter, randomized, open-label, active-controlled superiority trial evaluating treatment with either MBV or IAT in adult transplant recipients with CMV infection refractory, with or without resistance, to one or a combination of ganciclovir, valganciclovir, foscarnet, or cidofovir. Participants were randomized 2:1 to receive MBV or IAT for up to 8 weeks
- Costs were extracted from Singh et al. and inflated to USD 20222
- Range of the costs i.e., lower and upper values were calculated by applying (±) 25% arbitrarily
- Costs were calculated by combining ICU and non-ICU costs assuming the costs would vary according to the proportion of patients in each group
- Upper value extracted from Healthcare Cost and Utilization Project (HCUP) 2015
- Length of stay (LOS) estimates for 8 weeks were derived from the adjusted annual LOS estimates from the on-treatment period data
- Annualized mean LOS for MBV or the IAT arms was derived from a post hoc analysis of HCRU-related data collected as exploratory endpoints in SOLSTICE
- HCRU, defined as adjusted annualized mean LOS, in the model, was extracted from reported hospitalization stays in ICU and non-ICU, and overall hospitalizations related to their mean length of stay from on-treatment phase (8 weeks) data for both MBV and IAT treatment arms, respectively
- A probabilistic sensitivity analysis (PSA) was utilized to assess the impact of varying parameters on results
- Both costs of hospitalizations and LOS input parameters varied over the selected range of values
*MBV is an abbreviation of LIVTENCITY (maribavir).
HCRU Study: Assumptions1
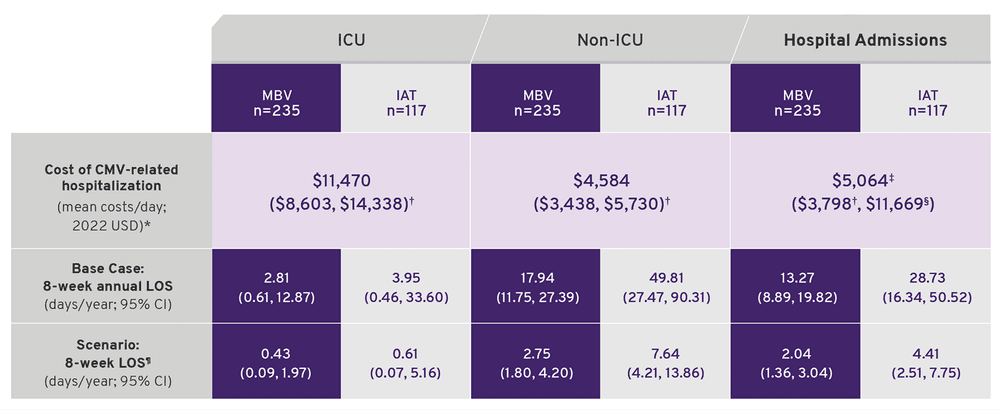
Base case and scenario analysis model inputs.
- *Costs were extracted from Singh et al.2 and inflated to USD 2022.
- †Range of the costs i.e., lower and upper values were calculated by applying (±) 25% arbitrarily.
- ‡Costs were calculated by combining ICU and non-ICU costs assuming the costs would vary according to the proportion of patients in each group.
- §Upper value extracted from Healthcare Cost and Utilization Project 2015.
- ¶LOS estimates for 8 weeks were derived from the adjusted annual LOS estimates from the on-treatment period data.
Base case analysis
- Model incorporated annualized estimates for length of stay (LOS) and costs based on the on-treatment phase (8 weeks) HCRU
- Mean per-patient-per-year (PPPY) HCRU costs for both MBV and IAT treatment arms were calculated using annualized mean LOS estimates derived from on-treatment phase LOS estimates for ICU, non-ICU, and overall hospitalization settings in SOLSTICE trial and multiplying them by direct per-day costs for CMV-related hospitalization
Scenario analysis
- Evaluated mean per-patient-per 8-week (PPP-8-week) costs for on-treatment phase (8 weeks) by re-estimating base case model inputs
- Inputs for average daily costs for ICU and non-ICU hospitalization were identical to the base case
- The point estimates and 95% confidence interval (CI) for 8-week LOS in days/year were derived from the trial data on file
- Mean PPP-8-week cost was calculated by multiplying the 8-week LOS with the per-day cost of CMV-related hospitalization
HCRU Study: Limitations1
- Results of clinical trial-related investigations may not generalize to broader CMV-infected patient populations in a real-world setting
- The cost inputs for this modeling analysis were derived from Singh et al. who examined CMV patients post-liver transplant, they may not be representative of the overall solid organ transplant (SOT) and hematopoietic stem cell transplant (HSCT) population with refractory CMV and could potentially underestimate the overall costs of CMV-hospitalization-related LOS2
- Costs associated with MBV and IAT were estimated based on generalized hospitalization costs in ICU and non-ICU settings and did not include direct costs of other healthcare resources or adverse events; nor indirect costs related to post-transplant patients with CMV infection
- The model inputs were derived from a study with an open-label design without blinding, which might have introduced bias
- Patient quality-of-life and adverse events costs were not included in the analysis
PPPY* Costs Were Lower for Maribavir Than IAT1
- IAT patients had costs that were nearly 2.5 times higher than those for MBV patients
- Overall hospitalization costs were driven by non-ICU hospitalizations, as there were few ICU hospitalizations in the SOLSTICE trial
- Costs for IAT patients were driven by the differences in annualized mean LOS between MBV (~13 days) and IATs (~29 days)
Base Case Analysis: PPPY Costs (2022 USD$)†
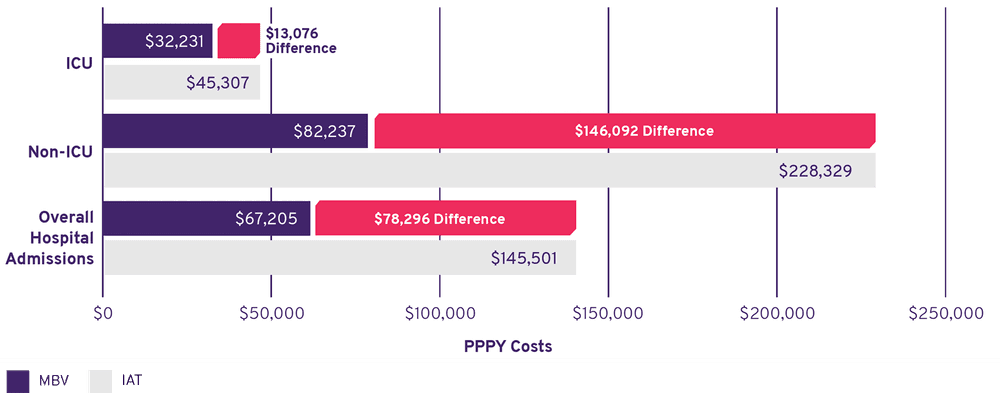
>99.99% of 1,000 model simulations demonstrated cost savings with MBV compared with IAT
- *Per-patient per-year (PPPY).
- †Prices are inflated to USD 2022; costs were not discounted.
Important Safety Information
Risk of Reduced Antiviral Activity When Co‑administered with Ganciclovir and Valganciclovir
LIVTENCITY may antagonize the antiviral activity of ganciclovir and valganciclovir by inhibiting human CMV pUL97 kinase, which is required for activation/phosphorylation of ganciclovir and valganciclovir. Coadministration of LIVTENCITY with ganciclovir or valganciclovir is not recommended.
Virologic Failure During Treatment and Relapse Post‑Treatment
Virologic failure due to resistance can occur during and after treatment with LIVTENCITY. Virologic relapse during the posttreatment period usually occurred within 4‑8 weeks after treatment discontinuation. Some maribavir pUL97 resistance‑associated substitutions confer cross‑resistance to ganciclovir and valganciclovir. Monitor CMV DNA levels and check for maribavir resistance if the patient is not responding to treatment or relapses.
Risk of Adverse Reactions or Loss of Virologic Response Due to Drug Interactions
The concomitant use of LIVTENCITY and certain drugs may result in potentially significant drug interactions, some of which may lead to reduced therapeutic effect of LIVTENCITY or adverse reactions of concomitant drugs. Consider the potential for drug interactions prior to and during LIVTENCITY therapy; review concomitant medications during LIVTENCITY therapy and monitor for adverse reactions. Refer to the full prescribing information of LIVTENCITY for important drug interactions.
Maribavir is primarily metabolized by CYP3A4. Drugs that are strong inducers of CYP3A4 are expected to decrease maribavir plasma concentrations and may result in reduced virologic response; therefore, coadministration of LIVTENCITY with these drugs is not recommended, except for selected anticonvulsants.
Use with Immunosuppressant Drugs
LIVTENCITY has the potential to increase the drug concentrations of immunosuppressant drugs that are CYP3A and/or P‑gp substrates where minimal concentration changes may lead to serious adverse events (including tacrolimus, cyclosporine, sirolimus and everolimus). Frequently monitor immunosuppressant drug levels throughout treatment with LIVTENCITY, especially following initiation and after discontinuation of LIVTENCITY and adjust immunosuppressant dose, as needed.
Adverse Reactions
The most common adverse events (all grades,> 10%) in subjects treated with LIVTENCITY were taste disturbance, nausea, diarrhea, vomiting, and fatigue.
Indication
LIVTENCITY is indicated for the treatment of adults and pediatric patients (12 years of age and older and weighing at least 35 kg) with post‑transplant cytomegalovirus (CMV) infection/disease that is refractory to treatment (with or without genotypic resistance) with ganciclovir, valganciclovir, cidofovir or foscarnet.
Please click for Full Prescribing Information.
1. Schultz BG, Bullano M, Paratane D, Rajagopalan K.Transpl Infect Dis. 2024;26(e14216):1-8. 2. Singh N, Winston DJ, Razonable RR, et al. Clin Infect Dis. 2021;73(9):e2739-e2745.