Cost-Effectiveness Study
Study 2: Cost-Effectiveness Model Study Design1
Methodology
- This model (appraised and approved by the Health Technology Assessment body, National Institute for Health and Care Excellence [NICE]) evaluated the cost effectiveness of MBV versus IAT for post-transplant refractory CMV infection
- A two-stage Markov model was designed using data from the SOLSTICE trial, real-world multinational observational studies, and published literature and included the following 3 health states for the first stage:
- Clinically significant CMV (csCMV) is CMV requiring treatment
- Nonclinically significant CMV (n-csCMV) is CMV that does not require treatment
- Dead
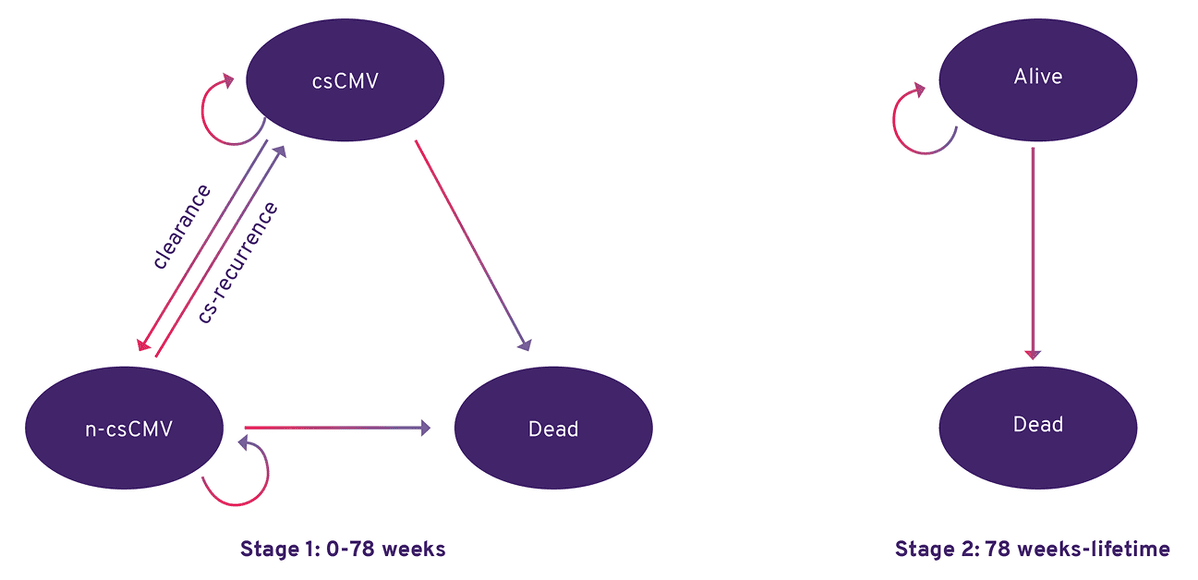
All patients begin in the csCMV health state and move to the n‑csCMV health state if they experience CMV DNA level <LLOQ (lower limit of quantification), or to the dead health state based on mortality data taken from the SOLSTICE trial.
Markov model structure.
cs-recurrence, clinically significant CMV recurrence.
This research article was funded by Takeda Pharmaceuticals. Several authors are employees or consultants of Takeda, Inc. or its affiliates and hold stock/stock options. One author has received consulting fees from Takeda.
Cost-Effectiveness Model Methods and Assumptions1
Methods:
- A standardized measure of health-related quality of life (QoL), EQ-5D, was used and the derived measures were applied in the model
- Direct medical costs include CMV-related and non-CMV-related hospitalizations, graft loss, and treatment-related costs
- These costs were estimated in 2022 USD and were derived using data from the Centers for Medicare & Medicaid Services Physician Laboratory Fee Schedule, the Healthcare Cost & Utilization Project 2022, the Medi-span Price RX 2022, and published literature
- The model used a lifetime horizon to capture all costs and benefits of treatment per the modeling guidelines, and the importance of capturing total survival time in a transplant population
- To determine cost-effectiveness, the health benefits/harms and costs of an intervention are compared with standard of care, where the output is the incremental cost-effectiveness ratio (ICER)
Clinical, cost, and health state inputs used in the model.
| Treatment arm | Probability of achieving CMV DNA level <LLOQ* at Week 8 (from SOLSTICE) |
|---|---|
| MBV | 55.7% |
| IAT | 23.9% |
| Inputs related to the health states | Value per 4-week cycle |
|---|---|
| Clinically significant CMV (csCMV) | |
| Quality of life (EQ-5D) during csCMV | 0.58ª |
| CMV-related hospitalization | $16,494 |
| Risk of hospitalization during csCMV | 25%ab |
| Cost of graft loss | $119,313 |
| Risk of graft loss during csCMV | 0.40% |
| Nonclinically significant CMV (n-csCMV) | |
| Quality of life (EQ-5D) during n-csCMV | 0.78ª |
| Non-CMV-related hospitalization | $3,006 |
| Risk of hospitalization during n-csCMV | 18%ab |
| Cost of graft loss | $119,313 |
| Risk of graft loss during n-csCMV | 0.13% |
- *LLOQ means lower limit of quantification, i.e. <137 IU/mL in 2 consecutive samples tested ≥5 days apart at end of Week 8.
- aWeighted for solid organ transplant (60%) and hematopoietic stem cell transplant (40%).
- bData on file.
Cost-Effectiveness Model Methods and Limitations1
Methods (Continued):
- For each treatment group, costs and quality-adjusted life years (QALYs) were estimated per 1,000 patients. An ICER was then calculated as the difference in mean costs between intervention (maribavir) and comparator (IAT), divided by the difference in mean QALYs between intervention and comparator
- A QALY is a measure of the value of a health outcome that is typically scored on a scale from 0 (death) to 1 (perfect or optimal health), integrating the life expectancy and treatment impact on morbidity of the compared interventions that may be used in HCEI2
Study Limitations:
- Use of data from the SOLSTICE trial, which may not capture nuances from real world clinical practice
- The cost of CMV hospitalizations was derived from the Healthcare Cost and Utilization Project, a national average of hospitalization costs for general CMV treatment and not necessarily for refractory CMV treatment
- This study included only patients with refractory CMV, which is more complex and costly to treat compared with general CMV, and the hospitalization costs in this model may be underestimated
- The QoL burden of intravenous vs oral administration was not included due to lack of data
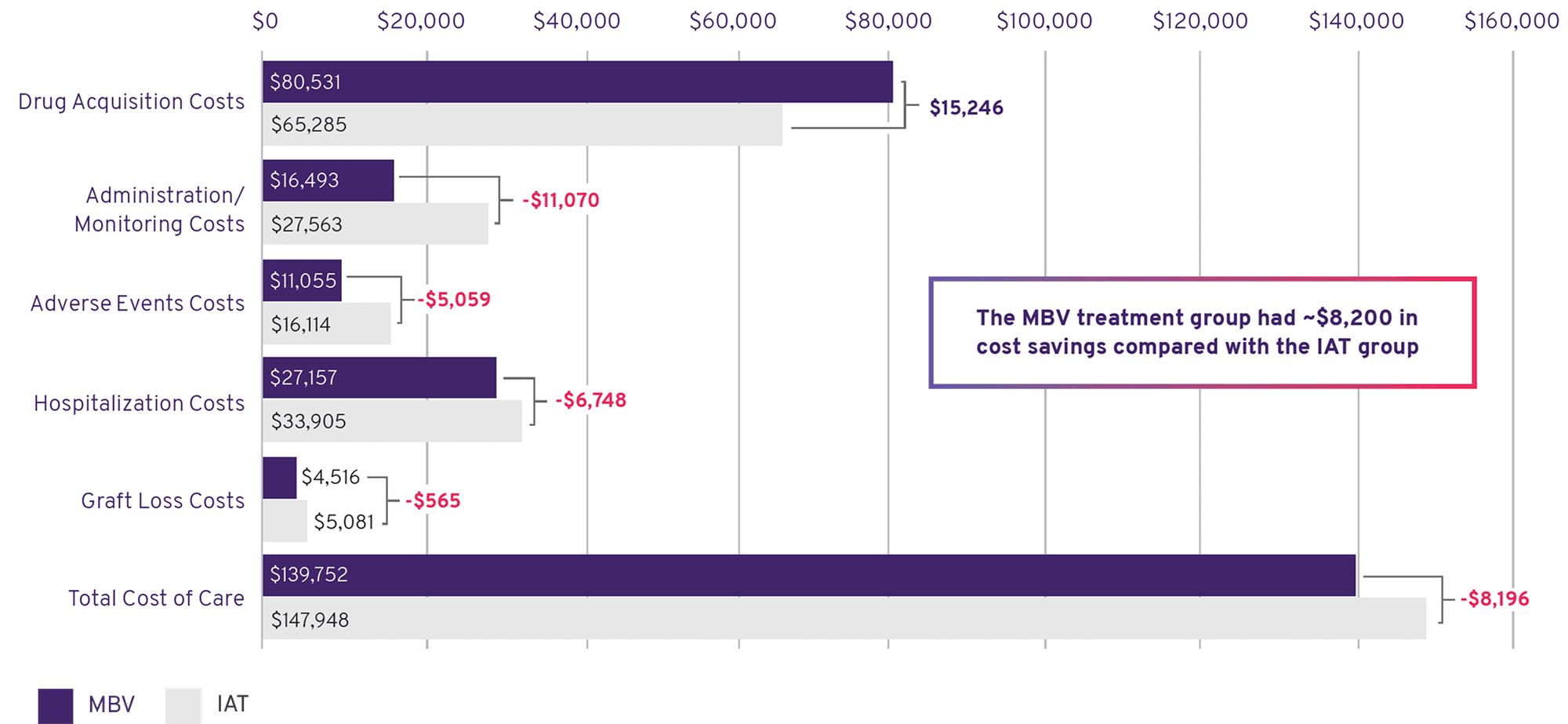
Maribavir Was More Cost Effective Than IAT1
- Drug acquisition costs for MBV were higher, but offset by reduced downstream costs and clinical results
- MBV had lower costs due to administration and monitoring, adverse events, hospitalizations, and graft loss
Total Costs Per Patient, Per Year: MBV and IAT
Important Safety Information
Risk of Reduced Antiviral Activity When Co‑administered with Ganciclovir and Valganciclovir
LIVTENCITY may antagonize the antiviral activity of ganciclovir and valganciclovir by inhibiting human CMV pUL97 kinase, which is required for activation/phosphorylation of ganciclovir and valganciclovir. Coadministration of LIVTENCITY with ganciclovir or valganciclovir is not recommended.
Virologic Failure During Treatment and Relapse Post‑Treatment
Virologic failure due to resistance can occur during and after treatment with LIVTENCITY. Virologic relapse during the posttreatment period usually occurred within 4‑8 weeks after treatment discontinuation. Some maribavir pUL97 resistance‑associated substitutions confer cross‑resistance to ganciclovir and valganciclovir. Monitor CMV DNA levels and check for maribavir resistance if the patient is not responding to treatment or relapses.
Risk of Adverse Reactions or Loss of Virologic Response Due to Drug Interactions
The concomitant use of LIVTENCITY and certain drugs may result in potentially significant drug interactions, some of which may lead to reduced therapeutic effect of LIVTENCITY or adverse reactions of concomitant drugs. Consider the potential for drug interactions prior to and during LIVTENCITY therapy; review concomitant medications during LIVTENCITY therapy and monitor for adverse reactions. Refer to the full prescribing information of LIVTENCITY for important drug interactions.
Maribavir is primarily metabolized by CYP3A4. Drugs that are strong inducers of CYP3A4 are expected to decrease maribavir plasma concentrations and may result in reduced virologic response; therefore, coadministration of LIVTENCITY with these drugs is not recommended, except for selected anticonvulsants.
Use with Immunosuppressant Drugs
LIVTENCITY has the potential to increase the drug concentrations of immunosuppressant drugs that are CYP3A and/or P‑gp substrates where minimal concentration changes may lead to serious adverse events (including tacrolimus, cyclosporine, sirolimus and everolimus). Frequently monitor immunosuppressant drug levels throughout treatment with LIVTENCITY, especially following initiation and after discontinuation of LIVTENCITY and adjust immunosuppressant dose, as needed.
Adverse Reactions
The most common adverse events (all grades,> 10%) in subjects treated with LIVTENCITY were taste disturbance, nausea, diarrhea, vomiting, and fatigue.
Indication
LIVTENCITY is indicated for the treatment of adults and pediatric patients (12 years of age and older and weighing at least 35 kg) with post‑transplant cytomegalovirus (CMV) infection/disease that is refractory to treatment (with or without genotypic resistance) with ganciclovir, valganciclovir, cidofovir or foscarnet.
Please click for Full Prescribing Information.
1. Schultz BG, Kotton CN, Jutla G, et al. J Med Virol. 2024;96(e29609):1-11. 2. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, et al. Drug and Device Manufacturer Communications With Payors, Formulary Committees, and Similar Entities: Questions and Answers. Guidance for Industry and Review Staff. Silver Spring, MD: U.S. Food and Drug Administration; June 2018. OMB Control No. 0910-0857.